Hello, Steve here again. It has been a month or so now at Friday Harbor and I’m still having a blast. Joe’s, Deanna’s, and my projects are going great. We are all starting to get results. While designing my experiment this spring one of my goals was to incorporate the scanning electron microscope (S.E.M.) into my research. I have always wanted to learn how to use one mainly because I think the pictures are really cool.
I did figure out how to include the S.E.M. by looking to see how morphological aspects of juvenile Oregonia gracilis affect their behavior. I am also interested in characterizing the different morphological features of the very young juveniles, as it has not been done before. Finally I am looking to see if there are any differences in setal density between different periods of O. gracilis’ life.
I learned how to use the S.E.M. last week. It is amazingly easy to use. If you can use a camera you can learn how to use the S.E.M. It only took about 5 minutes to master. The theory on how it works, on the other hand, is not so easy. Well, here are some pictures I took with it.
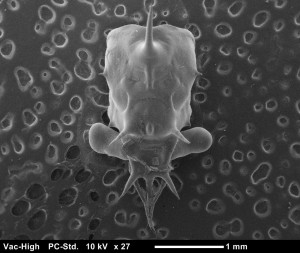
A carapace of an O.gracilis megalopa, the final larval stage of a crab before metamorphosis into a juvenile crab. They can look quite different between species. There are a few here that look similar but the way the spines are laid out distinguishes between them.
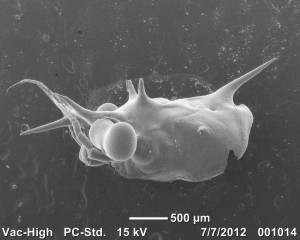
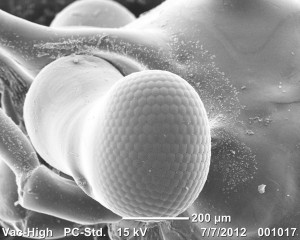
Compound eyeballs look tight.
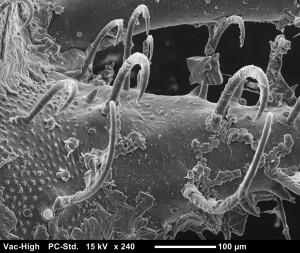
A carapace of a few day old juvenile O. gracilis. All the hair like projections are called hooked setae. These are how decorator crabs attach decoration to themselves. It is a mechanical attachment much like Velcro. Hooked setae are unique to the spider crab family Majoidae.
Here is a juvenile eye ball because it looks cool. It kind of has an eye brow of hooked setae. Pretty sweet.
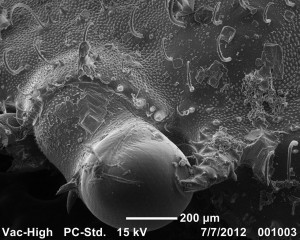
A close up of some of the setae. These ones are on the base of the rostrum.
Cheers,
Steve